Ok remember what we are hunting for cosmic rays. For more on cosmic rays click here. But the short story is that cosmic rays are basically charged particles from outer space. Specifically we are interested in stuff coming from the Sun so we can better understand solar activity. For why we should care click here.
The problem is that most cosmic rays from the sun don't make it all the way to the ground. Imagine a proton blasted off the surface of the sun that hurtles toward the Earth. Well pretty quick upon entering the atmosphere that proton is going to interact with an atom in the atmosphere so it doesn't make it all the way to the ground. But don't loose hope. When the proton (or other cosmic ray) blasts into the atom it can produce other particles. One of those particles is a neutron.
 Diagram at the CosRay Building of a cosmic ray producing other particles called an air shower.
Diagram at the CosRay Building of a cosmic ray producing other particles called an air shower.
But detecting neutrons can be tricky. They don't have an electrical charge. Detecting charged particles is easy. You can fill a tube with a gas, stick a charged wire in it, and ground the outside. Any charged particle entering the gas will want to go either to the charge or the ground. That creates a current which you can measure. That's basically how a Geiger Counter works. Anyway, we can't do that with neutrons. But there is an isotope of Boron that is really good at absorbing neutrons. It's called Boron 10. Anyway, when Boron 10 absorbs a neutron it breaks up. Specifically, it breaks up into Lithium 7 and Helium. Those are charged and they will also have enough energy to ionize some stuff around it creating even more charge (which is easy to detect.)
So the guts of the neutron monitor is a tube of a gas called Boron Trifluride (enriched so that most of the Boron is Boron 10). And there is a voltage across the tube so when that neutron comes along and makes the Boron break up then... wham! You have detected a neutron. But wait. There is another problem. There are neutrons around us all the time so you can't just leave the tube sitting on a table. You have to make sure only the neutrons we want get into the tube. The neutrons produced by cosmic rays are very high energy. The ones around us are low energy. So we screen the low energy ones out. Polyethylene will work for that. Low energy neutrons won't make it though that stuff but high energy ones will. But the high energy ones will probably also make it through the tube of gas. So we need a layer of polyethylene to block low energy neutrons but then we need some lead to absorb the high energy ones. The lead absorbs the high energy ones and becomes radioactive. It immediately gives off 8-12 low energy neutrons. THOSE neutrons hit the Boron 10 and cause it to break up into stuff that is easy to detect.
 The white plastic (polyethylene) screens out ordinary neutrons. The grey metal (lead) grabs the neutron and gives off more which the Boron in the tube then does its thing with.
The white plastic (polyethylene) screens out ordinary neutrons. The grey metal (lead) grabs the neutron and gives off more which the Boron in the tube then does its thing with.
Whew! Thats a lot but here's the basics. Cosmic ray hits the atmosphere and frees up a high energy neutron. The high energy neutron makes it to our detector and makes it through the polyethylene that keeps out regular neutrons. But then it gets to the lead and is absorbed. The lead gives off low energy neutrons which interact with the Boron which produces charged, easy to detect stuff.
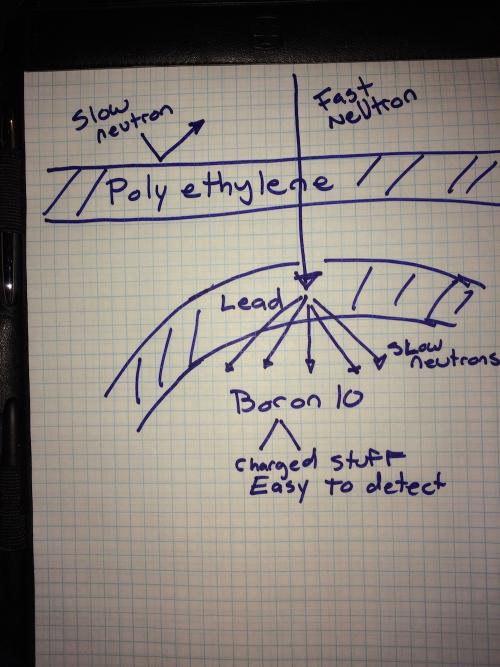 Cosmic ray hits stuff, make neutrons -> neutron makes it past polyethelyene into lead -> lead uses neutron to make more -> neutrons get absorbed by boron which fissions into detectable stuff
Cosmic ray hits stuff, make neutrons -> neutron makes it past polyethelyene into lead -> lead uses neutron to make more -> neutrons get absorbed by boron which fissions into detectable stuff

Comments
Add new comment