Closer Look: Dissolved Organic Carbon
Austin, Texas
May 25, 2019
Photo of the Day:
 Arctic surface waters on Alaska's North Slope. Image Source: Thomas Nash (High Country News).
Arctic surface waters on Alaska's North Slope. Image Source: Thomas Nash (High Country News).
As covered in my previous two posts on permafrost and the permafrost positive feedback loop, a great deal of organic material is being unlocked from thawing permafrost and remobilized. I wanted to learn more about this organic material.
What Is It?
Organic material in solution in rivers and streams is called Dissolved Organic Carbon, commonly abbreviated DOC. Being the product of decomposition of dead plants, animals, and microbes, it has a very complex makeup, one which is not comprehensively understood.1 As DOC composition varies geographically, I will focus specifically on Arctic DOC here. Mass spectrometry has indicated that Arctic DOC contains lignins, condensed aromatics, lipids, tannins, unsaturated hydrocarbons, peptides, carbohydrates, and amino sugars, along with many other aliphatics.2
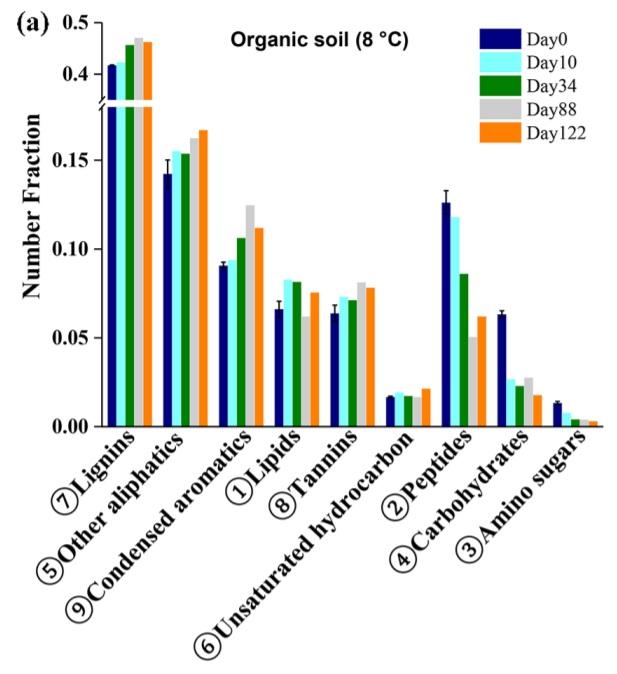 Organic composition of Arctic soil. Graphic from Chen, et al., 2018.
Organic composition of Arctic soil. Graphic from Chen, et al., 2018.
DOC Compound Classes
Let’s define these compound classes in more detail.
- Aromatics: Organic molecules that contain planar ring structures with exceptional stability due to a high degree of electron delocalization.
- Aliphatics: Organic compounds that are non-aromatic.
- Lipids: Fatty acids or their derivatives.
- Lignins: A class of organic polymers present in the structural tissue of plants. The commonality of lignins in DOC makes sense, as these polymers are extremely abundant in terrestrial plant material and very resistant to biodegradation.34
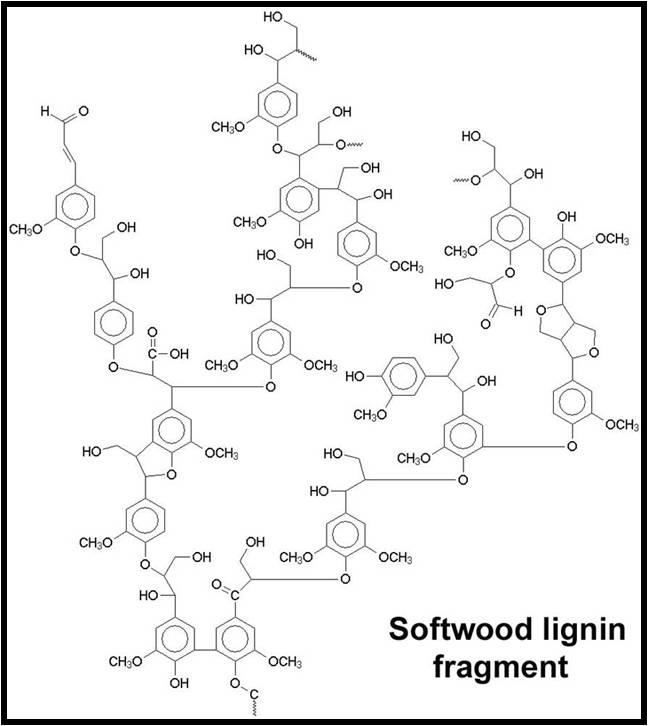 An example lignin polymer from softwood. Image Source: Roberta Farrell (University of Waikato, NZ).
An example lignin polymer from softwood. Image Source: Roberta Farrell (University of Waikato, NZ).
- Tannins: Complex biomolecules present in plant tissue that serve to bind and precipitate proteins. They play a role in plant defense against predation (as they give tissue a characteristic astringency) and are also involved in control of plant growth.4
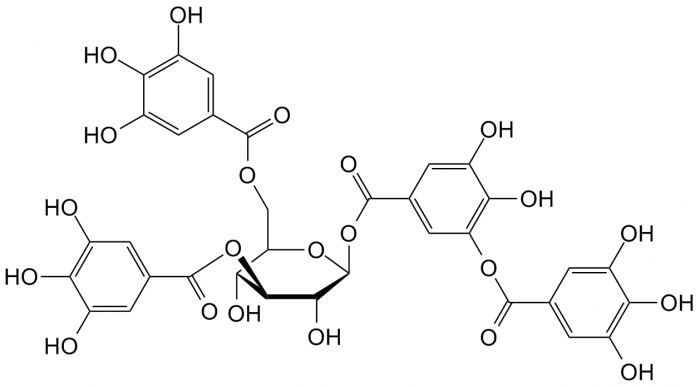 Tannic acid, a common tannin present in plants. Image Source: Wikipedia.
Tannic acid, a common tannin present in plants. Image Source: Wikipedia.
- Unsaturated Hydrocarbons: Organic compounds that contain pi bonds and/or rings.
- Peptides: Amino acid chains linked by peptide bonds.
- Carbohydrates: Sugars, starch, or cellulose
- Amino Sugars: Sugars in which one of the hydroxyl (-OH) groups has been replaced by an amine group (-NH2, -NHR, -NR2).
Why Is It Colorful?
As alluded to in my previous post, DOC often makes Arctic streams quite colorful (brown, yellow, even sometimes red). Where does the color come from?
Electrons within organic molecules form waves called molecular orbitals. When a compound absorbs energy, electrons are promoted (excited) to higher energy molecular orbitals. For most organic molecules, the absorbed energy is within the ultraviolet region of the spectrum. Accordingly, most organic molecules absorb ultraviolet light, reflect the entire visible spectrum, and appear white as a result.
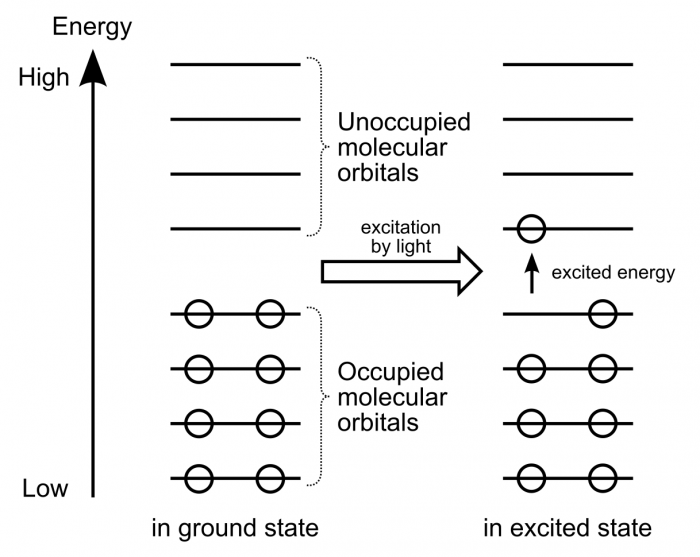 When a compound absorbs energy, electrons are promoted (excited) to higher energy molecular orbitals. Image Source: Wikipedia.
When a compound absorbs energy, electrons are promoted (excited) to higher energy molecular orbitals. Image Source: Wikipedia.
Compounds with higher degrees of electron delocalization have more overlapping regions of electron density. As a result, these compounds have more molecular orbitals. These molecular orbitals are more similar to each other in energy, and less energy is required to promote electrons to higher energy molecular orbitals.
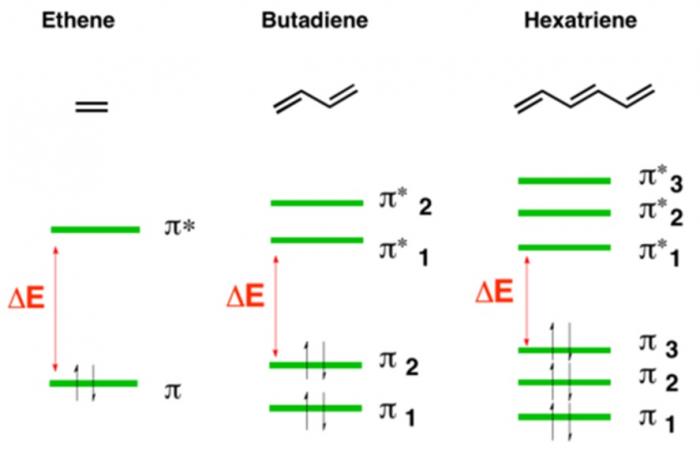 Of the above compounds, hexatriene has the greatest degree of electron delocalization. Accordingly, its molecular orbitals are the most similar to each other and it absorbs the least energetic light. Image Source: Master Organic Chemistry.
Of the above compounds, hexatriene has the greatest degree of electron delocalization. Accordingly, its molecular orbitals are the most similar to each other and it absorbs the least energetic light. Image Source: Master Organic Chemistry.
So, compounds with more electron delocalization absorb less energetic light. It follows that if a compound has enough electron delocalization, the energy absorbed is no longer in the ultraviolet range, but rather in the visible range. If a compound absorbs energy in the visible range, it no longer reflects that entire range back to the viewer. Instead of appearing white, it now appears colored!
Take a look at the amount of electron delocalization in the above lignin and tannin examples. It makes complete sense that these compounds are colorful.
 Many Arctic streams resemble dark tea due to high DOC concentration. Image Source: Dr. Rose Cory (University of Michigan).
Many Arctic streams resemble dark tea due to high DOC concentration. Image Source: Dr. Rose Cory (University of Michigan).
Other Examples
Here are some other neat examples of this same concept.
- Below is a graphic illustrating some of the more common organic dyes and pigments. Note the degree of electron delocalization in these compounds.
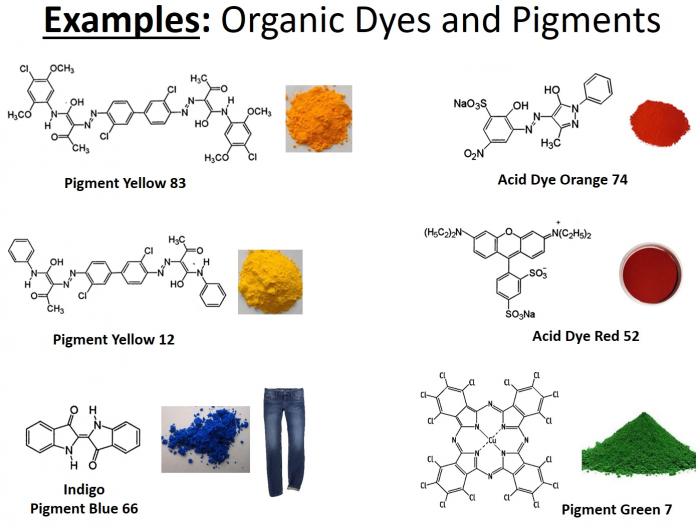 Common organic pigments and their associated colors.
Common organic pigments and their associated colors.
- Below is a great graphic prepared by Compound Interest illustrating the colorful compounds present in highlighters. Again, note the degree of electron delocalization in these compounds.
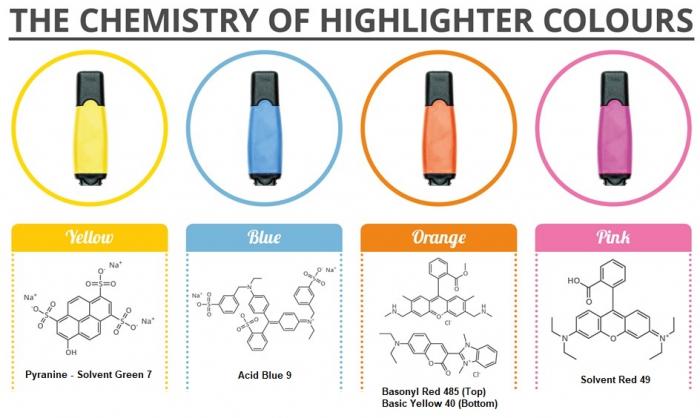 Example pigments contained in highlighters. Image Source: Compound Interest.
Example pigments contained in highlighters. Image Source: Compound Interest.
- One of the most commonly used acid-base indicators in phenolphthalein. This compound notably changes colors based on pH. In acidic conditions, it appears clear; in basic conditions, it appears pink. But why? The same concept applies here! The degree of electron delocalization in this compound changes based on whether it is protonated (acidic conditions) or deprotonated (basic conditions). Only in its deprotonated state is the electron delocalization great enough to allow for absorption in the visible spectrum (and accordingly, pink color!).
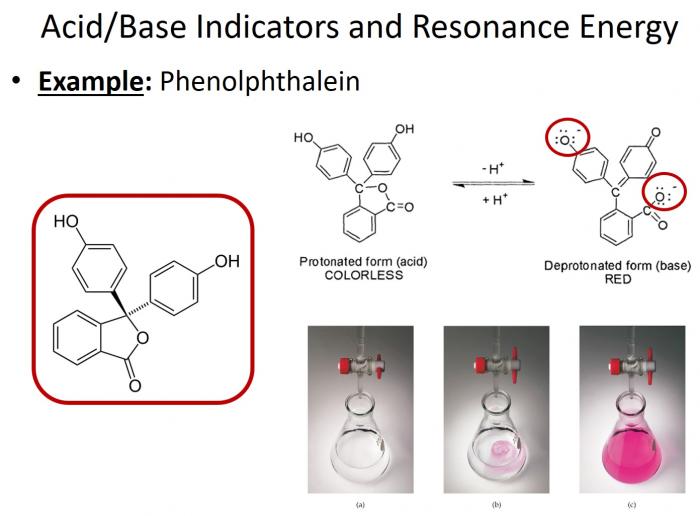 Phenolphthalein changes colors based on pH.
Phenolphthalein changes colors based on pH.
If you're interested, here's a presentation I use in my organic chemistry course with even more information on electron delocalization and color in organic chemistry:
Comment below!
-
Hawkes, Jeffrey A., et al. “Extreme Isomeric Complexity of Dissolved Organic Matter Found across Aquatic Environments.” Limnology and Oceanography Letters, John Wiley & Sons, Ltd, 15 Feb. 2018, aslopubs.onlinelibrary.wiley.com/doi/full/10.1002/lol2.10064. ↩︎
-
Chen, Hongmei, et al. “Molecular Insights into Arctic Soil Organic Matter Degradation under Warming.” Environmental Science & Technology, vol. 52, no. 8, 2018, pp. 4555–4564. ↩︎
-
Hillel, Daniel, and Jerry L. Hatfield. Encyclopedia of Soils in the Environment. Elsevier/Academic, 2005. ↩︎
-
Maier, Raina M., et al. Environmental Microbiology. Academic Press, 2000. ↩︎ ↩︎


Comments
Add new comment