Speed 4.1 knots (kts) Course 2.2° Location 72.46° N, 145.40° W Beaufort Sea, with CCG Cutter Louis S. St. Laurent Depth 3448 meters
SPECIAL FEATURE DISCUSSION:
(see previous journal for the questions.)
Cable tension increases as the coring rig is lowered because the cable itself adds ever more weight to the rig as more is reeled out.
In a core sample, the oldest layers are on the bottom. The law of superposition states that younger layers are deposited on older layers.
TODAY'S JOURNAL:
In the previous journal I described the process of gravity coring. After we recovered a gravity core from our target bump in the Beaufort Sea our marine technicians Pete DalFerro and Jenny White readied a more sophisticated sampling device called a piston core. The piston core is a bit more complex but has the potential of getting deeper samples from the sea floor because it is allowed to free fall in the last part of its descent, gaining speed to penetrate farther down.
The main coring rig is the same 2400-lb. weight and 4" steel pipe that we used for the gravity core, but this time two 10-foot pipe lengths were readied along with the core catcher and core cutter. But one additional device was placed at the tip of the pipe- the piston. The piston is a metal cylinder that fits snugly in the plastic core liner and is designed to keep the upper part of the sediment core more intact by acting as a sliding lid. Without the piston the upper layers can more easily separate and mix in the coring pipe.
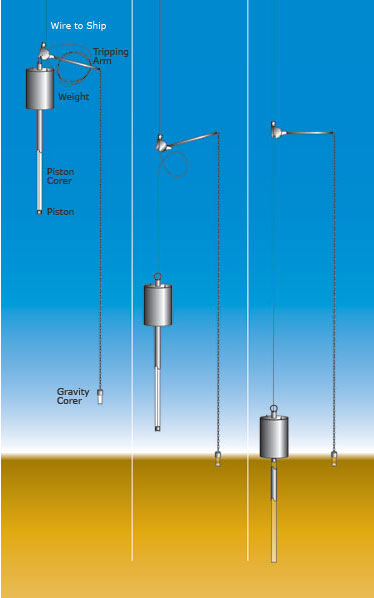 A diagram of the piston coring process from Woods Hole Oceanographic Institute
A diagram of the piston coring process from Woods Hole Oceanographic Institute
Image Source: http://www.whoi.edu/instruments/gallery.do?mainid=17288&iid=8087
The piston coring rig was suspended on a tripping arm mechanism with a 16-foot loop of slack cable attached to the weight. On the other end of the tripping arm was a small gravity corer with about 3 feet of coring pipe weighing about 250 pounds. The small gravity corer acts as a trigger for the tripping arm and when it hits the sea floor it releases the trigger, dropping the main piston corer hard into the sea floor. Then the whole rig is hoisted back to the surface. The short core from the trigger records surface layers and the main core goes as deep as possible.
 Here we see the tripping arm ready to receive the trigger weight. Note the coil of slack wire under Pete's arm. There is also a small cable lifting loop attached to the trigger mechanism.
Here we see the tripping arm ready to receive the trigger weight. Note the coil of slack wire under Pete's arm. There is also a small cable lifting loop attached to the trigger mechanism.
 Upon recovery we see the tripping arm has been released by the trigger, dropping piston corer into the sea floor.
Upon recovery we see the tripping arm has been released by the trigger, dropping piston corer into the sea floor.
 Chief scientist Brian Edwards fending off ice as the piston core rig was recovered. We were mostly in open water for the process but a band of drifting ice passed around us right as the gear was coming on board.
Chief scientist Brian Edwards fending off ice as the piston core rig was recovered. We were mostly in open water for the process but a band of drifting ice passed around us right as the gear was coming on board.
We were able to recover a much deeper sample this way, filling nearly the whole 20-foot length of pipe. As a very interesting surprise, a fizzing white substance was filling the core cutter at the tip of the coring rig. This bizarre material was gas hydrate! Gas hydrates form when there is the right combination of cold temperature and high pressure. Gas molecules (most commonly methane) get trapped inside of crystal cages of water molecules. Gas hydrates are stable as long as they stay under sufficiently high pressure and low temperatures, but by bringing it up to surface conditions the methane began to escape. It is kind of like having a two-liter pop bottle full of soda- there is a lot of carbon dioxide gas dissolved in the liquid but it stays dissolved when it is under pressure. But when you open the bottle and release the pressure bubbles form seemingly out of nowhere in the liquid and escape to the surface. Our gas hydrate sample was bubbling with methane as it escaped from the melting ice.
 Our first piston core returned a gas hydrate sample in the core cutter. The hydrate was about 20 feet beneath the sea floor. Once on deck it began fizzing with escaping methane.
Our first piston core returned a gas hydrate sample in the core cutter. The hydrate was about 20 feet beneath the sea floor. Once on deck it began fizzing with escaping methane.
http://
Gas hydrates turn out to be very widespread throughout the world's oceans and in permafrost areas. Studies suggest that there may be twice as much carbon stored in gas hydrates than in all other forms of fossil fuels on Earth combined. A growing concern among climate change scientists is the potential of releasing methane into the atmosphere if gas hydrates melt due to global warming. This could create a positive feedback loop because methane is a very effective greenhouse gas (a positive feedback tends to increase the process causing change.) So the worry is that more methane in the atmosphere could cause warming, which would melt more gas hydrates leading to more methane & more warming, etc. There are lots of other pieces to the global climate change puzzle but it was interesting to see this one component in person.
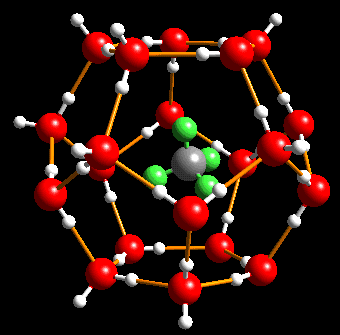 A model gas hydrate structure. At the middle is a methane molecule with one carbon (gray) and three hydrogen (green) atoms. The methane molecule is trapped by water molecules (red oxygen and white hydrogen atoms.) This structure is stable under sufficient conditions of high pressure but if those conditions are not met the methane will escape as the cage breaks down.
A model gas hydrate structure. At the middle is a methane molecule with one carbon (gray) and three hydrogen (green) atoms. The methane molecule is trapped by water molecules (red oxygen and white hydrogen atoms.) This structure is stable under sufficient conditions of high pressure but if those conditions are not met the methane will escape as the cage breaks down.
Image Source: http://peggy.uni-mki.gwdg.de/docs/kuhs/clathrate_hydrates.html
SPECIAL FEATURE:
Contrary to the law of superposition, the gas hydrate may not be the oldest layer in the core sample (even though it is at the bottom of the core.) Explain how this could be true.
That's all for now! Best- Bill

