Sea Ice In the Bay, Like Seeing an Old Friend
 As air temperatures dropped during the first weeks of 2020, areas of sea ice have formed in Kachemak Bay.
As air temperatures dropped during the first weeks of 2020, areas of sea ice have formed in Kachemak Bay.
Approximately two months after the Akademik Fedorov left the ice edge and I said my goodbyes to the sea ice of the Central Arctic Ocean, I woke up to see a strange dull patch of water along the shore of Kachemak Bay. It reminded me of grease ice. The weather has been cold here since the last few days of 2019. Over the past 3 weeks, air temperatures have fluctuated mostly between 0 and 20 degrees Fahrenheit (-6 to -17 degrees Celsius). The result -- sea ice!
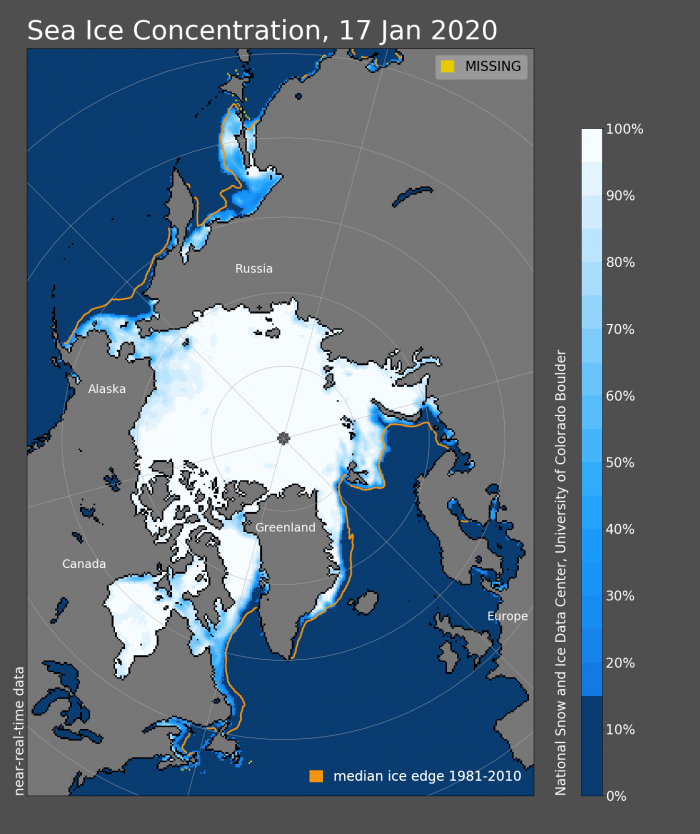 This graphic from the National Snow and Ice Data Center shows sea ice concentration as of January 17. Note the small area of light blue in southcentral Alaska (just to the left of the letter
This graphic from the National Snow and Ice Data Center shows sea ice concentration as of January 17. Note the small area of light blue in southcentral Alaska (just to the left of the letter
When I asked Marc Oggier, a sea ice scientist from the University of Alaska Fairbanks, what sea ice is, his answer was straightforward. Sea ice is frozen seawater. I pondered that. Did that mean I'd encountered sea ice before in the waters around my hometown? I guess so, though I'd never really thought of it that way.
Growing up in Homer, every few winters we would see chunks and slushy layers of ice form in shallow, low wave-action areas as the air temperature dipped. And sometimes, the cold temperatures persisted and the ice grew thicker, more stable. About a decade ago, we had a cold snap and the surface water in the Homer Harbor froze more than a few inches thick. The radio and newspapers published notices from the harbormaster, warning people against skating on the unstable ice of the harbor. The winter of 1988-1989, the inside portion of Kachemak Bay froze solid leaving people living in Bear Cove trapped, unable to navigate back to Homer via boat. Some local residents remember seeing blue mussels (a type of mollusk, like a clam) dead on the beach after the hard freeze.
 A layer of sea ice has formed in the Homer Harbor this winter, despite the fact that the docks rise 15-25 feet vertically with each high tide.
A layer of sea ice has formed in the Homer Harbor this winter, despite the fact that the docks rise 15-25 feet vertically with each high tide.
 Beautiful patterns of sea ice, open water, and snow form along the shore in the Homer Harbor as the tides ebb and flow.
Beautiful patterns of sea ice, open water, and snow form along the shore in the Homer Harbor as the tides ebb and flow.
Recently, a local mariner recalled working in nearby Cook Inlet many years ago. Sea ice was common during some of those winters. His captain would pull the boat alongside a floe and cut the engines. Strong tidal currents would push the sea ice and boat along at the same speed. Sometimes, if it seemed safe, they'd use the ladder to climb down from the boat and walk on the ice.
 In shallow Mud Bay, chunks of sea ice are broken, moved, and frozen together every tidal cycle.
In shallow Mud Bay, chunks of sea ice are broken, moved, and frozen together every tidal cycle.
Here in Kachemak Bay, we rarely have sea ice that stable. With some of the most extreme tides in the world, water pours twice a day into the shallow areas where ice is likely to form, breaking up the ice and moving it around. The tides drive the movement of massive amounts of water. Pieces break apart and are piled on top of each other as the water moves in and out, creating strangely beautiful forms. For ice that freezes farther offshore, Kachemak Bay is still fairly narrow; currents are likely to eventually slam the ice into rocky cliffs or cast it ashore on beaches. At the same time, these currents and tides and high winds mix the water and drive upwelling of relatively 'warm' deeper water. While the water at the surface loses heat to the cold atmosphere, this deeper water can actually carry heat into the upper boundary layer between the ocean and atmosphere, where the water freezes into ice and the ice melts back into liquid.
While freshwater freezes at about 32 degrees Fahrenheit (0 degrees Celsius), the salinity of sea water means that it freezes closer to 28 degrees Fahrenheit (-1 or -2 degrees Celsius). In the estuarine waters of Kachemak Bay, where fresh and saltwater mix, the freezing point is probably a degree warmer than pure ocean water. Here, I imagine it to be a near continuous cycle when the air temperatures are in this range. Cold air freezes the ice from above, while warmer water melts it from below. How long does any one piece of ice last in these dynamic, mixing waters of Kachemak Bay? I'm not sure of the answer.
 "Slushy waves carry sea ice and break it into smaller pieces as the tide rises and falls.
"Slushy waves carry sea ice and break it into smaller pieces as the tide rises and falls.
On beaches exposed to bigger waves, some pieces of ice can be seen on shore. They seem to last longer here, where they are exposed on all sides to the cold air. But as high tide pulls them into the water, the pounding of the waves reduces them to a slush. This slushy of battered sea ice joins with new ice crystals forming in the seawater -- frazil ice -- and also perhaps snow from shore. The semi-frozen mixture slows and dampens the movement of the waves. The waves appear to be in slow motion, and there is a soft shushing sound as the slush meets the shore. For the past two weeks, this is what we've experienced here in Kachemak Bay. As the air temperature continues to hover at less than 20 degrees Fahrenheit, the cover of sea ice has crept more thoroughly across shallow parts of the Bay.
For me, it has been a special reminder of the time I spent in and on the sea ice of the Central Arctic Ocean. Because of the dynamic movement of water from tides and waves, most of the ice here is a softer, more fragile form than what I saw in the Arctic. The thickest pieces are at most 0.5 meters (1.5 feet) thick and look like a flattened snowman that iced over a bit. They are fairly soft and slushy and I barely trust them with my body weight. When inspected closely, they are strikingly different from what I encountered in the Arctic.
 Chunks of ice that formed elsewhere in Kachemak Bay are cast ashore by waves at the end of the Homer Spit. Note the pancake ice-like formations just offshore.
Chunks of ice that formed elsewhere in Kachemak Bay are cast ashore by waves at the end of the Homer Spit. Note the pancake ice-like formations just offshore.
But in the Harbor, I saw what looked like fresh sheets of grey-white ice. I tried to break through it with a bucket to bring a piece home for a closer look. The ice was too strong. Nearby, a boat powered through the ice. It was just a large skiff, but the sound of the thin ice banging along its aluminum hull reminded me of the sounds I'd hear from my bunk as the Fedorov ploughed through the Arctic sea ice. And along some of the shores, I've seen slushy pancake ice that reminds me of what we saw as we entered the ice for the first time on the Fedorov.
 Smooth sheets of grey ice have formed in the Homer Harbor. There are places where smaller pieces of ice, broken by the movement of the docks due to tides, is scattered across this layer.
Smooth sheets of grey ice have formed in the Homer Harbor. There are places where smaller pieces of ice, broken by the movement of the docks due to tides, is scattered across this layer.
Unlike the sea ice in the Central Arctic Ocean, even the thickest ice here doesn't seem to have the typical brine channel structure. I think the constant movement of water and the not-so-cold temperatures make it all granular ice. In granular ice, the ice crystals are arranged randomly. As sea ice continues to grow, the ice crystals begin to form in a more predictable pattern: they are arranged vertically. These ice crystals grow over time. Some of them can be more than a centimeter (0.5 inch) in diameter, and up to dozens of centimeters/inches in length. This columnar ice, and the salty brine channels that form within it, make up an important habitat for a whole community of sea ice organisms. A core taken from sea ice in the Arctic will often have a layer of granular ice at the top, and a much thicker layer of columnar ice below. If you were to take a core of our Kachemak Bay ice, I think it would be granular all the way through.
 Slushy ocean water is slowly pushed ashore into snow as the tide rises on a wave-exposed beach on the Homer Spit.
Slushy ocean water is slowly pushed ashore into snow as the tide rises on a wave-exposed beach on the Homer Spit.
The ice in Kachemak Bay won't last long. In the Central Arctic Ocean, people speak of multi-year ice. This refers to ice that has survived, or stayed frozen, through more than one year. Multi-year ice is not a thing in Kachemak Bay or Cook Inlet. Any remnants of sea ice that make it to spring will quickly melt as air temperatures creep to 40, 50, 60, 70, and, more common in recent years, 80 degrees Fahrenheit. It is likely that the sea ice here won't even last to spring. The ten-day forecast is predicting air temperatures between 2 and 26 degrees Fahrenheit. But at some point in the next couple of weeks, air temperatures will probably crest above the 28 or 29 degree mark. At that point, our sea ice will quickly melt away. The Bay will be all liquid again. We will be left with photos and memories of the frozen seascape, glittering in the long sunsets of winter. And I will be left wondering. How does the sea ice, however briefly it is present, impact the phytoplankton, algae, and animals that live in the Bay? What do you think?
 This slushy ocean water seems to be a combination of snow and frazil ice. It glitters in the sun.
This slushy ocean water seems to be a combination of snow and frazil ice. It glitters in the sun.
Education Extension A recipe to make your own sea ice, from Marc Oggier
How to make sea ice: 1) Pour sea water into a bucket or container at least 2 feet deep 2) Make sure sides and bottom of container are insulated (you can use Styrofoam or foam camping pads for this) 3) Leave some space at the top of the container, the water will expand as it freezes 4) Put in freezer, or outside if it will be consistently well below freezing 5) From time to time, slowly stir the ice so you form granular ice at the top (this is simulating wind) 6) Then cover the ice with plastic wrap 7) Allow to continue to freeze for a few days 8) Take out of container 9) If you want, carefully slice with a saw into smaller chunks
What can you learn with your artificial sea ice? Measure the volume of the liquid water (before or after it is sea ice) and compare it to the volume of the frozen sea ice.
Use a thermometer to take the temperature of sea ice at different depths. If possible, it is useful to drill horizontally into the sea ice at these depths and then insert your thermometer/temperature probe.
Compare the salinity of the sea ice (you'll need to melt it) with the salinity of the liquid water left at the bottom of your container. Compare this to melted freshwater ice. If your ice/seawater comes from a clean area, do a taste test.
Look at the ice with polarized glasses. This should show the arrangement of ice crystals. Compare this to a freshwater sample frozen in the same way.
Put a few drops of food coloring on the top and bottom of the ice. You may be able to see this food coloring flow into the brine channels in the sea ice!


Comments
Add new comment