Science Update
Last night we traveled from the Bransfield Strait in the Antarctic Sound to reach sampling stations in the Weddell Sea. The Antarctic Sound is also known as "Iceberg Alley" because the mountains surrounding the sound area are covered with ice sheets and glaciers. Icebergs regularly break off of these ice features and float freely in the waters of the sound.
 This large ice shelf in Antarctica Sound could be the origin of many of the icebergs that give this location its nickname 'Iceberg Alley'.
This large ice shelf in Antarctica Sound could be the origin of many of the icebergs that give this location its nickname 'Iceberg Alley'.
 Smaller icebergs and bergy bits (small floating ice pieces) float through Antarctica Sound.
Smaller icebergs and bergy bits (small floating ice pieces) float through Antarctica Sound.
The Weddell Sea is on the eastern side of the Antarctic Peninsula and is an area known for deep water and lots of ice. The Weddell Sea was discovered in 1823 by James Weddell. Is was originally named George IV Sea, but was changed in 1900 to honor Weddell. The Weddell Sea is also the location where Sir Ernest Shackleton found his ship, the Endurance trapped in pack ice for months before being destroyed. Thankfully, we did not experience the same amount of ice and made it to our sampling station without incident. We spent the morning filtering samples from Day 7 of the incubation experiment and used the rosettes to sample seawater at this station in the afternoon.
The Iron Story Continued
In yesterday's journal, I introduced the chemical oceanography concepts related to this research cruise. I did not, however, give you the full story. There is more to being a diatom here in the Southern Ocean. Not only are you living in an area historically known for low concentrations of iron, you are also living in water. Confused?? So was I until I learned more about the two different forms of iron found in ocean water. Without going into too much detail, iron can exist in oxygenated water in two different oxidation states: Fe(II) and Fe(III). Due to the non-acidic, oxygenated state of the seawater, most of the iron is in the form of Fe(III). This is a problem for diatoms (and other microscopic organisms) because Fe(III) in non-soluble. This means that organisms can not easily take in Fe(III) when it is in water. The Fe(III) usually falls to the deeper parts of the ocean and is, therefore, unavailable for diatoms to use for photosynthesis. So, not only is there a limited amount of iron, there is a limited amount of the type of iron/Fe(II) that diatoms can use. It sounds like the makings for a soap-opera if you ask me! But wait! There is a way for diatoms to use the Fe(III) - and here's where the biology saves the day!
Ligands to the Rescue
There are biological molecules called ligands that have parts (functional groups) that bind to metal to form what scientists refer to as a complex. When these ligands and metals combine, the process is known as chelating (pronounced key-lating). The combination of a ligand and a metal can sometimes change the properties of the metal. Iron chelating compounds called siderophores are found in bacteria, fungi and grasses. These siderophores have an affinity (attraction) to Fe(III). When they combine, the Fe(III) becomes a soluble form that can be used by organisms. In the diagram below, the green and yellow lines represent the ligand molecules. The greenish-tan organisms are diatoms and the blue ovals are bacteria. Some of the Fe(III) is not associated with ligands and is sinking to the bottom. The other Fe(III) is bound to ligands and is now available for use. You may notice, however, that diatoms do not have ligands. Even though they have a high iron requirement, diatoms do not produce siderophores of their own.
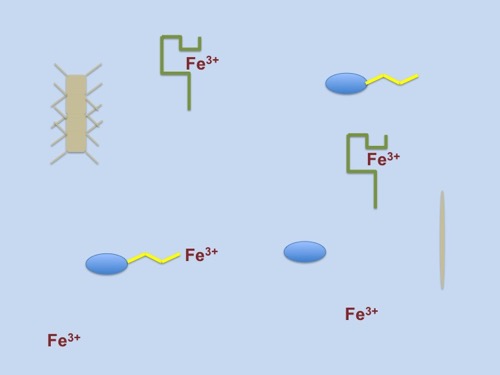 The yellow and green 'molecules' in the picture represent siderophores. These siderophores bind to Fe(III) creating a complex that can be used by organisms.
The yellow and green 'molecules' in the picture represent siderophores. These siderophores bind to Fe(III) creating a complex that can be used by organisms.
Scientists hypothesize that diatoms and the bacteria have a synergy (relationship) that benefits both organism. The diatoms are able to use the Fe(III) bound to ligands on the bacteria's membrane and the bacteria receive nutrients from the diatoms. All of the scientists on this research cruise are interested in studying the ligands present in the Southern Ocean. Whether from the chemical or biological angle, the science team is interested in learning more about this synergistic relationship and how the diatoms are acquiring iron necessary for photosynthesis.
The Great Debate
To wrap up today's journal, I am looking for help to try and determine an answer to tonight's great debate. During an amazing sunset photography session in Duse Bay, many crew members and science team members photographed a cloud with a distinct glow - like an iridescence. No other cloud in the sky had the same coloration. After some recent and extensive conversations, we have decided that the cloud is one of two types: a lenticular cloud or a nacreous cloud. The problem is - no one on board is experienced enough to know for sure. Do you have an answer? Do you know someone who could help end the great debate? Add your thoughts to the comments or forward this journal to someone who may know. There are a number of folks aboard the RVIB Palmer that would appreciate your help!
 The bright white cloud in the upper left hand portion of the picture was seen by the RVIB Palmer crew on September 20th. The question remains whether this cloud is a lenticular cloud or a nacreous cloud.
The bright white cloud in the upper left hand portion of the picture was seen by the RVIB Palmer crew on September 20th. The question remains whether this cloud is a lenticular cloud or a nacreous cloud.

Comments