The I-series is a set of connected lakes and streams that feed into each other and ultimately end up in Toolik Lake. These bodies of water cover an expanse of Arctic Tundra watershed that includes tussock tundra, heath tundra, and shrub tundra.
 Snow on the tundra. Photo by DJ Kast
Snow on the tundra. Photo by DJ Kast
The I-series is executed three times a year for the Arctic Long Term Ecological Research (LTER) Program. This LTER has been sampling this watershed regularly for 16 years. The LTER and Dr. Byron Crump’s lab work together to take not only environmental data but also microbial data as well.
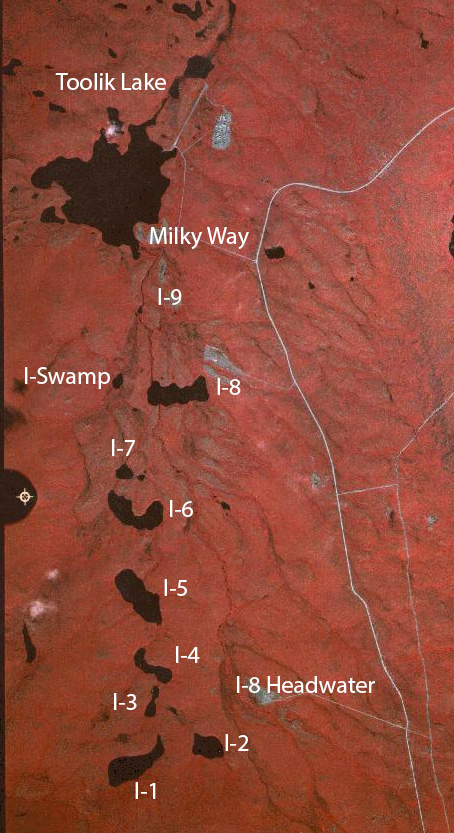 A map of the I-series. Photo by Toolik Field Station
The I-series people!
A map of the I-series. Photo by Toolik Field Station
The I-series people!
We assembled three teams to go out into the field: a lakes team, a microbe team, and land/water team.
The Lakes team:
- Dan White
- Sam Finnerty
- Brecia Douglas
- Dr. Kim Bernard
The Land/Water team:
- Jason Dobkowski
- Chris Cook
- Dr. George Kling
Microbe team:
- Dr. Byron Crump
- Johanne Albrigtsen
- DJ Kast
- Graham Stewart
Lakes Team
The Lakes team took a small yellow raft out into the middle of each of the I-series lakes and took a gamut of measurements and water samples.
 A photo of the Lake team's raft. Photo by DJ Kast
A photo of the Lake team's raft. Photo by DJ Kast
 Sam and Brecia rowing on the Lake. Photo by DJ Kast
Sam and Brecia rowing on the Lake. Photo by DJ Kast
 Treking through the Arctic Tundra with the Lakes raft. Photo by DJ Kast
Treking through the Arctic Tundra with the Lakes raft. Photo by DJ Kast
They took out a CTD (Conductivity, Temperature, Depth), to measure the conductivity (freshwater salinity, essentially) and temperature at certain depths.
 Brecia carrying the CTD to the boat. Photo by DJ Kast
Brecia carrying the CTD to the boat. Photo by DJ Kast
Then a large water sampling bottle was sent to that particular depth to collect water samples for the microbe team.
 Sam with the water sampler. Photo by DJ Kast
Sam with the water sampler. Photo by DJ Kast
A secchi disk was used at each site to measure the water clarity. They also towed for plankton in each lake.
Land/Water Team
The Land/Water team measured stream flow rate in the inlets and outlets of each of the lakes.
 Chris Cook in Steam I6 outlet. Photo by DJ Kast
Chris Cook in Steam I6 outlet. Photo by DJ Kast
They used hand held sensors to measure temperature, conductivity and pH.
 Jason and Chris filtering their outlet/inlet water samples. Photo by DJ Kast
Jason and Chris filtering their outlet/inlet water samples. Photo by DJ Kast
 Jason in his element by the Lakes in bug gear. Photo by DJ Kast
Jason in his element by the Lakes in bug gear. Photo by DJ Kast
They collected water to measure chemistry, and they collected special “gas samples” to measure the concentration of CO2 and methane in the water.
 Dr. George Kling taking gas samples of the lakes. Photo by DJ Kast
Dr. George Kling taking gas samples of the lakes. Photo by DJ Kast
Microbe Team
The Microbes team got water from Lakes and Land/Water teams and then filtered the water to collect all of the microorganisms on handy little “sterivex” filter capsules. They added a buffer to the filters to preserve the DNA for later. They also used some of the water to measure the growth rate of bacteria.
 DJ practicing with the chaulk gun to make filtering easier when the filter gets clogged. Photo by DJ Kast
Helicoptering into the field!
DJ practicing with the chaulk gun to make filtering easier when the filter gets clogged. Photo by DJ Kast
Helicoptering into the field!
Lake I-1 is approximately 5 miles away from Toolik and to save time on the hiking, we took a helicopter ride with pilot Keaton Molt to get there.
 Helicopter by Lake I5 preparing to bring the groups to I6 headwaters and back to Toolik Camp. Photo by DJ Kast
Helicopter by Lake I5 preparing to bring the groups to I6 headwaters and back to Toolik Camp. Photo by DJ Kast
Trip back home:
 An aerial shot of our Toolik Basecamp. Photo by DJ Kast
Samples to Collect:
An aerial shot of our Toolik Basecamp. Photo by DJ Kast
Samples to Collect:
These are the list of sites and depths at which water is collected for each of the experiments.
 Water Sample Sites and Depths. Photo by DJ Kast
Water Sample Sites and Depths. Photo by DJ Kast
 A photo of the i8 inlet. Photo by DJ Kast
A photo of the i8 inlet. Photo by DJ Kast
 I6 headwaters. Photo by DJ Kast
I6 headwaters. Photo by DJ Kast
 i3 into i4 outlet. Photo by DJ Kast
i3 into i4 outlet. Photo by DJ Kast
 i4 into i5. Photo by DJ Kast
i4 into i5. Photo by DJ Kast
 i4 with the Lakes team taking samples in the middle of the lake. Photo by DJ Kast
i4 with the Lakes team taking samples in the middle of the lake. Photo by DJ Kast
 A panorama of the i4 lake. Photo by DJ Kast
A panorama of the i4 lake. Photo by DJ Kast
 i6 outlet with Chris Cook. Photo by DJ Kast
i6 outlet with Chris Cook. Photo by DJ Kast
- These are preferred depths – VERIFY THE DEPTH. Only sample for DNA. No Bacteria Production.
Packing List:
- 8 x 2L brown plastic bottles
- 7 x Thermoses for Bacteria Production incubations
- DNA sampling kit (for each day of I-series)
- Small cooler with 3-4 frozen blue ice blocks
- 4 x 140cc syringes with tape marking 140ml
- 3 caulking guns that fit the 140cc syringes
- 10 pair gloves
- 40 x GP Sterivex filters
- 40 x male luer lock plugs (sterile)
- 1 x tray of Cha-Seal
- DEB syringe filled with DEB
- 2 x 0.2um pore size syringe filter
- DEB holder (plastic tube with cork)
- Extra DEB syringe
- Extra 40ml of DEB (in cooler)
- 2x Sterivex Organizers
Bacteria Production kit:
- Umbrella
- Leucine
- p200 Pipette
- p200 Pipette tips
- 10 ml sterile plastic pipette
- Pipette bulb
- 20 ml plastic scintillation vials (4 per sample)
- Scintillation vial caps and septa (4 per sample)
- 50% TCA
- 3 ml syringes, needle
- Gloves
- Thermometer (instant read)
- Thermoses (as many as needed)
- Tupperware holder for supplies, one for samples with scintillation vial rack
DAY 1
- Give Lakes group 4 x 2L bottles, 2 x thermos (for I1 and I2)
- Give Land-Water group 1 x 2L bottle, 1 x thermos (for I8HW)
- Fly to I3 (south-east side between inlet streams) a. One person walks to I2 and I1 to get water from Lakes group (see #1) b. Land-Water group will bring water from I8HW c. Give Land-Water group 1 x 2L bottle, 1 x thermos for I6HW Inlet d. Give Lakes group 2 x 2L bottles, 1 x thermos (for I3) e. Collect and process I1-I3 and I2-I3
 Dr. Byron Crump collecting water from i2 into i3. Photo by DJ Kast
Dr. Byron Crump collecting water from i2 into i3. Photo by DJ Kast
f. Receive and process I1, I2, I3 and I8HW.
 Johanne collecting water samples. Photo by DJ Kast
Johanne collecting water samples. Photo by DJ Kast
- Give Lakes group 2 x 2L bottles, 1 x thermos (for I4)
 Here I am collecting water for Graham's bacteria production in a thermos. Photo by DJ Kast
Here I am collecting water for Graham's bacteria production in a thermos. Photo by DJ Kast
- Walk to I4, process I4
- If we go to I5, give Lakes group 2 x 2L bottles, 1 x thermos (for I5) a. Walk to I5, process I5 and I4-I5 b. Give Lakes group 2 x 2L bottles, 1 x thermos (for I6HW Lake)
- If we go to I6HW Lake directly from I4 a. Give lakes 2 x 2L bottles, 1 x thermos (for I6HW Lake) b. Process I6HW Lake and I6HW Inlet
 Boating through i6 headwaters. Photo by DJ Kast
Boating through i6 headwaters. Photo by DJ Kast
c. Give Lakes 2 x 2L bottles, 1 x thermos for I5 8. Fly to the lake we didn’t do and process it.
DAY 2
- Give Lakes group 4 x 2L bottles, 2 x thermos (for I6 and I7)
- Give Land-Water group 1 x 2L bottle, 1 x thermos (for I5-I6)
- Fly to I6 Inlet West. a. Collect and process I6 Inlet West b. Receive I5-I6 from Land-Water group and process them
- Walk to I7 a. Receive I6 and I7 samples from Lakes group and process them b. Give Lakes group 4 x 2L bottles, 2 x thermos for I8 and I-Swamp
- Walk to I8 Inlet a. Collect and process I8 Inlet b. Walk to Lakes group and get I8 and/or I-Swamp samples and process them.
- Walk to I-Swamp Inlet a. Collect and process I-Swamp Inlet.
- Walk to collect and process: a. I8 outlet b. I7-I9 c. I8-I9 d. MWL e. Toolik Inlet
Bacteria production instructions in the field
Graham Stewart is our bacteria production person.
 Stewart in bug gear and doing his bacteria production experiment. Photo by DJ Kast
Stewart in bug gear and doing his bacteria production experiment. Photo by DJ Kast
He uses the following methods to calculate how much carbon is being turned into bacterial biomass essentially it measures bacterial growth. The amino acid Leucine is added as a tag to label the new proteins that are being made, which is synonymous with new growth.
 Graham Stewart working his production experiments. Photo by DJ Kast
Graham Stewart working his production experiments. Photo by DJ Kast
- Collect 2 L bottle of water (for subsequent DNA processing), one thermos with temperature of collected water.
- Write down temperature and time of collection.
- Inject 10 mL sample into each of 4 scint vials (20 mL). a. Use 10 mL pipette with bulb
- Kill fourth vial with 1 mL of 50% TCA, swirl.
- Inject all 4 vials with 50 uL Leucine working solution, cap, shake.
- Incubate 4 vials for 2 hours in the thermos at temperature +/- 1 degree from in situ temperature.
- At 2 hours, using 3 mL syringe with needle attached, inject 1 mL 50% TCA to vials 1-3. Shake.
- Return to lab for filtering, counting.
Dr. Byron Crump is looking at microbial diversity of the lakes and streams in the Arctic Once the water is collected, the following process occurs to remove the microbes from the water onto a filter that can be tested in the lab.
- Pull plunger out of syringe.
- Attach syringe to Sterivex filter.
- Pour sample into the back of the syringe to ~140 mL line.
- Insert plunger, set volume to 140 ml, and push sample through sterivex filter.
- Remove sterivex filter (or leave the filter on and turn the three-way stopcock on the syringe to allow plunger removal).
- Repeat. Aim to get a minimum of 500 mL and up to 1L of sample water through the filter.
 Byron filtering water for microbes. Photo by DJ Kast
Byron filtering water for microbes. Photo by DJ Kast
- Force all water out of filter using the syringe filled with air.
- Shake last few drops out of filter forcefully.
- Stab the filter outport into the Cha-seal
- Inject 1 mL DNA Extraction Buffer (DEB) into the filter inport. If using a needle, do not pierce the white filter inside. (You can put all the DEB buffer in a 60 cc syringe and just use it repeatedly for each sample). Be careful not to add more than 1 ml.
 Here I am adding DEB buffer to the sterivex filter. Photo by DJ Kast
Here I am adding DEB buffer to the sterivex filter. Photo by DJ Kast
- Close inport with a male luer plug or a 3 cc syringe. Be sure it is seated tightly. The syringe sometimes slips out.
- Freeze ASAP, store at –80ºC. If in the field, put in a small cooler with ice packs.
 Our finished field samples. Photo by DJ Kast
Arctic Tundra Lounging and Napping
Our finished field samples. Photo by DJ Kast
Arctic Tundra Lounging and Napping
 Always looking fashionable in the field. Model Dr. Byron Crump. Photo by DJ Kast
USC and YSP Polar Flag
Always looking fashionable in the field. Model Dr. Byron Crump. Photo by DJ Kast
USC and YSP Polar Flag
 Arctic Tundra USC flag. Photo by DJ Kast
Arctic Tundra USC flag. Photo by DJ Kast
 Vermont's Ms. Baker classroom's Polar Science YSP Flag with TA Dawson Ray. Photo by DJ Kast
Vermont's Ms. Baker classroom's Polar Science YSP Flag with TA Dawson Ray. Photo by DJ Kast
 Posting with the YSP flag in front of the landing helicopter. The YSP Polar flag belongs to Ms. Kim's class at 32nd St School (YSP TA Anna Duan). Photo by DJ Kast
Posting with the YSP flag in front of the landing helicopter. The YSP Polar flag belongs to Ms. Kim's class at 32nd St School (YSP TA Anna Duan). Photo by DJ Kast
 32nd St school's YSP Flag belonging to Ms. Kim's and YSP TA Anna Duan's class. Photo by DJ Kast
32nd St school's YSP Flag belonging to Ms. Kim's and YSP TA Anna Duan's class. Photo by DJ Kast

Comments