Balloon Launch
Every Wednesday, an Ozone balloon is launched from the South Pole Station. I was lucky enough to observe one of these balloon launches!
 Gavin holding the balloon getting ready for the launch.
Gavin holding the balloon getting ready for the launch.
Along for the ride are several scientific instruments aiming to collect data at various altitudes. There are some meteorological instruments, such as a thermometer and a barometer, as well as an Ozone probe. Between the pressure readings from the barometer and a GPS tracker, the altitude of the balloon at any given moment is known.
This equipment is placed in an insulated, Styrofoam box. Right before launch, the box is heated using what looks like a fancy hair dryer. It’s important that the equipment in the box doesn’t freeze in the upper atmosphere, but over-heating can also be damaging. A delicate balance must be struck.
 Gavin holds what looks like a fancy hair dryer used to heat the styrofoam box of equpiment. It's important that this instruments don't freeze as they goes into the upper atmophere, but over-heating them can also be damaging. Dave reads temperature data inside the box on a computer in the other room until it's exactly the desired temperature. Then the sytrofoam lid is quickly placed on to keep the heat from escaping.
Gavin holds what looks like a fancy hair dryer used to heat the styrofoam box of equpiment. It's important that this instruments don't freeze as they goes into the upper atmophere, but over-heating them can also be damaging. Dave reads temperature data inside the box on a computer in the other room until it's exactly the desired temperature. Then the sytrofoam lid is quickly placed on to keep the heat from escaping.
For 5-10 minutes, the Styrofoam box of equipment sits outside taking some surface-level data. Then, it’s attached to the balloon and, like a scene straight out of the movie “Up,” off she goes!
 The balllon is all ready to be launched! The white, styrofoam box on the bottom contains all of the instruments necessary to take data on the balloon's ascent and descent. The orange parachute helps to slow down the descent.
The balllon is all ready to be launched! The white, styrofoam box on the bottom contains all of the instruments necessary to take data on the balloon's ascent and descent. The orange parachute helps to slow down the descent.
 Seconds after letting go, the balloon is already high in the air.
Seconds after letting go, the balloon is already high in the air.
 Up, up and away to an altitude of ~36km!
Up, up and away to an altitude of ~36km!
The whole flight takes about 3 hours during which time the balloon reaches a maximum altitude of ~36km. The balloon pops and the Styrofoam box of equipment floats down to the ground. Data can be collected both on the ascent and descent so it’s important to have a parachute to slow it down. The balloon eventually lands about 20-30km away from the station, and it is never recovered (except one which happened to land only 3km away due to some interesting winds).
After the balloon launch, I was able to talk to Gavin & Dave, two guys from the National Oceanic & Atmospheric Administration (NOAA), who are experts at balloon launches, the ozone, and the ozone hole. A special thanks to Mr. Miller (no we’re not related) from Washington-Lee High School for sending me some guiding questions for me to ask!
Here’s what I learned:
Ozone Probe
What is the Ozone anyway? The Ozone is a layer of inorganic molecules known as trioxygen (O3) in our upper atmosphere. Ozone molecules appear as a pale blue gas, are distinctively pungent, and play a major role in Earth’s atmosphere. You see, Ozone molecules absorb UV radiation coming from the sun. This is especially important to us humans because UV light is what causes sunburns, and even worse skin cancer!
Attached to the weather balloon is an Ozone probe.
 Gavin holding the Ozone instrument before it's put in the sytrofoam box to get ready for launch.
Gavin holding the Ozone instrument before it's put in the sytrofoam box to get ready for launch.
The Ozone probe consists of a little tube that leads to two containers. One container is filled with highly concentrated sodium nitrate, the other with slightly less concentrated sodium nitrate. The tube takes in ozone molecules from the atmosphere that then interact with the sodium nitrate. Due to the different concentrations in each container, a small current begins to flow between them. The current output is proportional to the amount of Ozone molecules sucked in through the tube. This data is then sent via radio signal to the computers back at the station to be recorded.
The Ozone Layer
At the base of our atmosphere is what’s known as the Troposphere layer. Nearly 90% of all molecules in Earth’s atmosphere reside in this layer. As the balloon ascends through the Troposphere, the temperate decreases.
But, at some point the temperature begins to increase. This is the start of the Stratosphere.
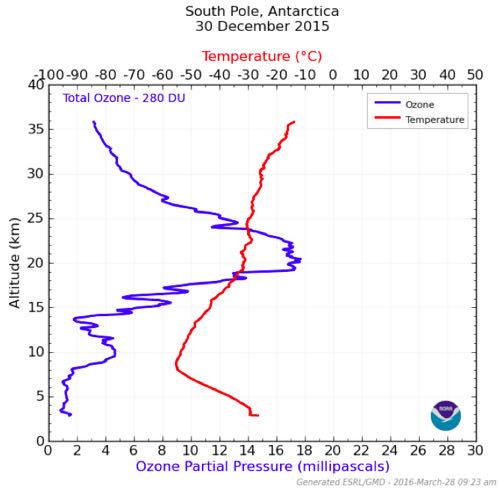 Looking at the red temperature data, you'll notice a decreasing temperature all the way up to about 8km of altitude. Then the temperature starts to increase. This is where the Troposphere transitions to the Stratosphere. This corresponds to the blue Ozone data where you see an increase in Ozone particles around 8km. (Credit: LTJG Gavin D. Chensue, NOAA - Antarctic Ozone Depletion)
Looking at the red temperature data, you'll notice a decreasing temperature all the way up to about 8km of altitude. Then the temperature starts to increase. This is where the Troposphere transitions to the Stratosphere. This corresponds to the blue Ozone data where you see an increase in Ozone particles around 8km. (Credit: LTJG Gavin D. Chensue, NOAA - Antarctic Ozone Depletion)
The reason the temperature increases in the Stratosphere is because of the presence of Ozone molecules. As Ozone molecules absorb UV rays, they give off heat. Above the United States, the transition from Troposphere to Stratosphere is at an altitude of about 12km. At the South Pole, this transition occurs at an altitude of about 8km. This is why airplanes must fly so low here – the vast majority of the molecules in the South Pole atmosphere are below 8km.
The Ozone Hole
You may have heard about the Ozone hole on the news several years ago. This is referring to a hole in the Ozone primarily over Antarctica. This hole gets particularly big during September and October, during which time Ozone balloon launches are increased from once per week to every other day.
What created this hole? Why is it over Antarctica?
When a Chlorine molecule in the atmosphere interacts with an Ozone molecule, it effectively destroys the Ozone molecule. In fact, one Chlorine molecule will destroy about one million Ozone molecules before running out of steam.
There are two reasons why Chlorine molecules are especially prevalent in the Antarctic atmosphere, and thus create the Ozone hole.
- Antarctica is cold. The temperature affects how Chlorine molecules interact with Ozone molecules, increasing the rate of destruction.
- Around Antarctica is the Southern Ocean that stretches completely around the globe. Winds formed here create a polar vortex that surrounds the continent of Antarctica. This polar vortex serves as a barrier, holding Chlorine molecules over Antarctica. These Chlorine molecules then go to work destroying Ozone molecules.
Effects of the Ozone
Although the hole in the Ozone is over Antarctica, it’s not just Antarctica that’s impacted. The Antarctic Ozone hole sucks Ozone molecules from nearby Australia and New Zealand, especially around early November. Unfortunately this is late spring/early summer for the Aussies and Kiwis so everyone’s headed to the beach. Without the Ozone molecules there to absorb UV rays, skin cancer rates in Australia and New Zealand have skyrocketed – a huge public health concern!
Here at the South Pole, we’re not at quite as high of a risk of skin cancer. For one, our skin is almost always covered with our Extreme Cold Weather gear. Additionally, the angle of the sun in the sky never reaches more than 23.5 degrees (see this journal for details on why) which makes its rays less intense. Nonetheless, sunscreen is still highly encouraged here!
In the 1990s, we realized this Ozone hole existed and started to take measures to fix this problem. Strict regulations on the use of chemicals that negatively affect the Ozone (particularly Chlorofluorocarbons often found in refrigerants) were put in place. At this point, we think the Ozone hole is getting smaller, but we’re still monitoring it to be sure. Current estimations predict that the Ozone hole will return to pre-1970 levels around 2080.
Ozone Data
Two of Ms. Brasfield’s Chemistry students at Washington-Lee High School, Alina and Hannah, have been working hard to design an experiment for me to take to the South Pole. After a few iterations, they arrived on the idea of measuring Ozone molecules.
 James modeling the Ozone Test Kit that students Alina and Hannah sent with me to the South Pole. Inside are small strips that are meant to be placed outside. The strips change color according to how many Ozone molecules are detected.
James modeling the Ozone Test Kit that students Alina and Hannah sent with me to the South Pole. Inside are small strips that are meant to be placed outside. The strips change color according to how many Ozone molecules are detected.
I took some preliminary data in Omarau, New Zealand using the Ozone strips. I observed no color changes, leading me to question if the strips were showing the lowest ozone reading or no reading at all.
Today, I took data at South Pole. I observed a definitive change in color this time, with all four trials falling in the <90 micrograms of ozone per cubic meter category.

It is important to note some of the conditions of when these strips are accurate are tough to meet here. The directions say to avoid direct sunlight (there’s 24 hours of sunlight here), avoid wind (there’s usually wind here), and measure only in the humidity range of 30-60% (it’s consistently ~0% humidity here). If these conditions are not met, the reading might be an underestimate. To take the above data at the South Pole, I placed all four trials in a shaded area mostly shielded from the wind right outside the station.
After, I returned to Dave & Gavin (the NOAA guys) to see if the readings I got were consistent with the data they take on the Ozone. They directed me to this website: esrl.noaa.gov/gmd/. On this site, there is data from balloon launches like the one I observed available to anyone! I hope this online resource can help you out with your experiment, girls!
I plan to take a few more samples when I’m back in Christchurch just to see what the New Zealand Ozone looks like.
Overall, I’ve been really impressed by Alina and Hannah’s ability to think creatively as they worked to design a compelling experiment. Their perseverance and dedication serve as an inspiration to all of us who do science.


Comments
Add new comment